Prostamax Research Overview
Prostamax is a short regulatory peptide modeled after naturally occurring signaling molecules found in prostate tissue. Peptides are small chains of amino acids that act as biological signaling messengers, helping cells respond to stress, inflammation, and age-related changes.1,2
In preclinical research (animal and laboratory studies), Prostamax has been shown to influence inflammatory signaling, oxidative stress, fibrotic remodeling, and epithelial repair within prostate tissue.3-6 These effects appear to support healthier tissue structure and function, particularly in models involving chronic irritation or inflammation.
Important regulatory clarification: Prostamax is a research-use-only (RUO) peptide. It is not approved by the FDA for the diagnosis, treatment, prevention, or cure of any disease, including benign prostatic hyperplasia (BPH). Findings described below are derived from non-human and experimental research models only.
Key Actions (Preclinical Research Context)
- Supports epithelial repair and tissue organization3,4
- Modulates cytokines and immune-cell infiltration5,6
- Reduces fibrotic (scar-like) tissue remodeling6,7
- Protects against oxidative stress8,9
- Influences age-related gene-expression patterns1,2,10
Key Clarification
Reducing inflammation and fibrosis may reduce the inflamed, swollen component of prostate tissue, but this is not the same as reversing the hyperplastic (cell-overgrowth) component that defines BPH.11,12
🧬 What Is Prostamax?
Prostamax belongs to a class of peptides often referred to in the scientific literature as organ-specific bioregulators.¹˒² These peptides are studied for their ability to influence cellular signaling within a specific tissue by helping normalize pathways involved in inflammation, repair, and age-associated decline.
Its tetrapeptide core (four amino acids) is small enough to enter cells and interact with regulatory systems that influence gene activity, immune signaling, and tissue maintenance.²˒¹⁰
Core Research Areas (Preclinical)
- Epithelial Integrity: Supports repair of the glandular lining in experimental models3,4
- Inflammatory Control: Reduces excessive immune signaling5,6
- Anti-Fibrotic Effects: Limits abnormal collagen accumulation6,7
- Microvascular Stability: Supports small blood vessels⁸
- Epigenetic Modulation: Influences gene activity associated with aging¹˒²˒¹⁰
Molecular Details
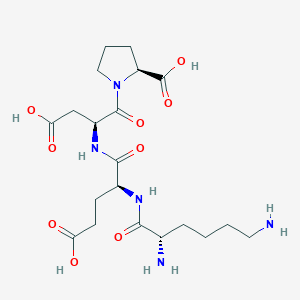
- Sequence: Lys-Glu-Asp-Pro (KEDP)
- Molecular Weight: ~487.5 g/mol
- Molecular Formula: C20H33N5O₉
- PubChem CID: 9848296
Prostamax and Epithelial Tissue Repair
In animal and tissue-culture studies, Prostamax supported recovery of prostate epithelial cells following inflammatory or chemical stress.3,4
Observed findings included:
- Increased epithelial thickness
- More orderly cellular layering
- Reduced immune-cell infiltration
- Preservation of normal glandular architecture
These findings indicate support for tissue quality and resilience in research settings, not direct reduction of prostate cell mass or organ size.
Prostamax, Inflammation, Fibrosis, and the Two-Layer Model
Executive Summary
Prostate enlargement often reflects two overlapping biological processes:
- Hyperplasia – a long-term increase in prostate cell number (the defining feature of BPH)¹¹˒¹²
- Inflammation & congestion – swelling caused by immune activity, fluid accumulation, vascular dilation, and fibrosis5-7
Current Prostamax research addresses primarily the second process, not the first.
1. Anti-Inflammatory Effects
Preclinical studies show Prostamax can:
- Reduce TNF-α and IL-6 (inflammatory cytokines)5-6
- Decrease immune-cell infiltration6
- Reduce edema and vascular congestion6-7
Why this matters:
Inflamed tissue occupies more volume than non-inflamed tissue.¹¹ Reducing inflammation may therefore reduce the swollen component of prostate enlargement in experimental models.
Critical limitation:
This does not remove the additional cells produced by hyperplasia.
2. Reduction of Fibrotic Remodeling
Prostamax has demonstrated the ability to:
- Reduce excess collagen deposition6,7
- Normalize fibroblast activity7
- Preserve tissue flexibility
Fibrosis contributes to stiffness and functional restriction but does not define BPH itself.11,12
3. Microvascular Support
Prostamax supports endothelial stability, oxygen delivery, and protection against oxidative vascular stress in experimental systems.8,9
Prostamax and Immune / Cellular Signaling
Prostamax influences cellular stress-response pathways by:
- Reducing reactive oxygen species8,9
- Enhancing antioxidant enzyme activity9
- Supporting epithelial stem-cell niches4
- Stabilizing age-related gene expression 1,2,10
These effects help maintain healthier cellular behavior but do not reverse established hyperplasia.
Prostamax and Oxidative Stress Regulation
Oxidative stress contributes to inflammation, fibrosis, and cellular dysfunction in aging prostate tissue.8,9
In experimental models, Prostamax has been shown to improve antioxidant defenses and mitochondrial function.8-10
Prostamax and Hormonal Signaling — Defined Boundary
Benign prostatic hyperplasia is primarily driven by androgen signaling, especially dihydrotestosterone (DHT).¹²˒¹³
Prostamax has not been shown to inhibit 5-α-reductase, reduce DHT levels, or block androgen receptors.
🔬 BPH Reversal Criteria — Scientific Clarification
While reduced prostate volume, decreased hyperplastic cell density, suppressed androgen signaling, and improved urinary flow are well-established criteria for demonstrating BPH reversal, these endpoints have not been evaluated in controlled studies of Prostamax. Existing research has focused primarily on inflammation, fibrosis, and tissue health rather than androgen-driven hyperplasia.11-13
🔭 Future Research Context
Controlled studies evaluating prostate volume, hyperplastic cell density, androgen signaling, and urinary flow metrics would be required before any claims related to BPH reversal could be considered.11-13
Summary
- Inflamed prostate tissue occupies more volume than non-inflamed tissue¹¹
- Reducing inflammation may reduce the swollen component of enlargement
- BPH is defined by durable cellular overgrowth, not swelling alone¹²
- Prostamax research addresses inflammation, fibrosis, oxidative stress, and tissue maintenance
- Prostamax has not been shown to prevent, treat, or reverse BPH
References
- Khavinson V, Lin’kova N. Short peptides as regulators of gene expression and aging. Biogerontology. 2012;13(4):367-374.
- Khavinson V, Tendler S, Ashapkin V, et al. Peptide regulation of chromatin structure and gene expression. Russ J Genet. 2017;53(4):371-382.
- Popovich IG, Anisimov VN, Khavinson V. Peptide bioregulators and epithelial regeneration. Biogerontology. 2017;18(4):573-589.
- Lin’kova N, Khavinson V. Peptide regulation of epithelial stem-cell niches. Bull Exp Biol Med. 2016;161(3):388-391.
- Khavinson V, Diatlova A, et al. Peptide complexes in inflammation control. Adv Gerontol. 2015;5(1):45-52.
- Grigoriev EI, Khavinson V. Effects of prostate peptides in chronic prostatitis models. Urologiia. 2012;(2):43-47.
- Lin’kova N, Khavinson V. Bioregulation of fibrosis by short peptides. Biochem (Mosc). 2018;83(3):345-352.
- Anisimov VN, Khavinson V. Peptide regulation of oxidative metabolism. Biogerontology. 2010;11(2):139-149.
- Lin’kova N, et al. Antioxidant effects of regulatory peptides. Mol Cell Biochem. 2018;444(1-2):123-131.
- Khavinson V, Ashapkin V. Epigenetic mechanisms of peptide bioregulators. Curr Aging Sci. 2019;12(2):78-87.
- Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia an inflammatory disease? Eur Urol. 2007;51(5):1202-1216.
- Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res. 2008;20(S3):S11-S18.
- McConnell JD, et al. The role of androgens in the pathogenesis of BPH. J Urol. 2003;170(2):S8-S12.






